Examining the role of cytochrome oxygenases in austocystin D-mediated cytotoxicity
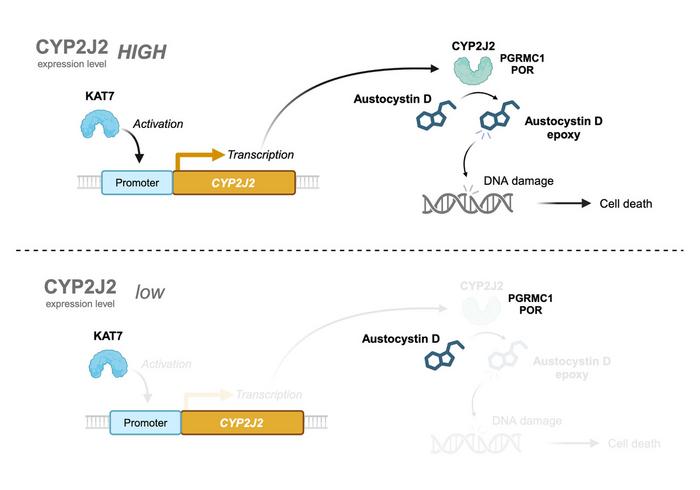
image:
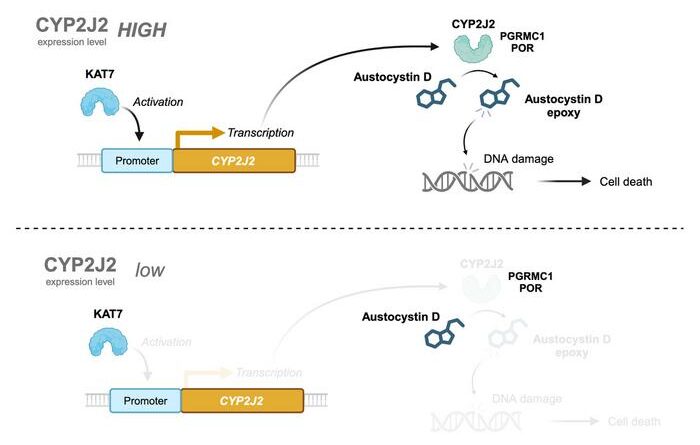
Previous studies have shown that austocystin D is oxidized by cytochrome P450 (CYP) oxygenases which then causes DNA damage and suppresses cell proliferation. In this study, we demonstrated that CYP2J2 is required for austocystin D-mediated cellular injury; lysine acetyltransferase 7 (KAT7) promotes the transcription of CYP2J2, and PGRMC1 and POR can help CYP2J2 enzymatic activity. Austocystin D is not easily oxidized in cells with low expression of CYP2J2, and therefore does not adversely affect cell proliferation in such cells.
sight Again
Credit: Professor Mahito Sadaie of Tokyo University of Science
Austocystin D, a natural compound produced by fungi, is known for its cytotoxic effects and anticancer activity in various cell types. It shows strong activity even in cells that produce proteins associated with multidrug resistance, attracting significant global research interest. Austocystin D promotes cell death by damaging their DNA, a process that may depend on cytochrome P450 (CYP) oxygenase enzymes. In particular, austocystin D showed greater activity against cancer cells with increased CYP expression. However, the specific role and activity of the CYP2J2 enzyme in austocystin D cytotoxicity remains to be determined.
Against this background, a team of researchers from the Tokyo University of Science in Japan succeeded in revealing the mechanism of action of austocystin D involving CYP2J2. The research team included Mrs. Yukiko Kojima and Professor Mahito Sadaie, both from the Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science, Japan, among others. They used a new research method that combined gene silencing techniques with growth restriction experiments in cell models. Their pioneering findings have been published online in a journal Cancer Science on July 15, 2024.
Initially, researchers used data from the Japanese Foundation for Cancer Research 39 to examine the relationship between austocystin D sensitivity and CYP expression in cancer cells. By examining the genetic markers in these cells, they found that the expression of a specific CYP gene, namely CYP2J2, was positively correlated with sensitivity to austocystin D. Furthermore, Sadaie and his team confirmed their findings. with correlation studies, they used 11 human osteosarcoma (OS) – four cancer cell lines, which confirmed the relationship between CYP2J2 expression levels and austocystin D sensitivity.
To reveal the main mechanism of action of austocystin D, researchers used U-2 OS cells, which express high levels of CYP2J2. They observed that U-2 OS cells treated with austocystin D showed significant DNA damage, while HOS (bone cancer) cells with low expression of CYP2J2 were less sensitive to combination. Prof. Sadaie thanks the joint efforts of researchers for discovering the CYP2J2 mechanism and components, “This research work was a joint project involving the Department of Applied Biological Science, Tokyo University of Science, and Dr. Ishikawa’s group from Kyoto University, Dr. Shin-ya’s group from the National Institute of of Advanced Science and Technology, and the groups of Dr. Dan and Dr. Tomida from the Japan Cancer Research Association.
In addition, the researchers conducted a series of experiments to evaluate the effects of overexpression and reduction of CYP2J2 on austocystin D-mediated cytotoxicity. Their results confirmed that excessive CYP2J2 enhanced the cytotoxic effects of austocystin D, while its reduction resulted in reduced sensitivity to austocystin D and significantly reduced cytotoxicity in cancer cells.
To better understand the genes involved in regulating CYP2J2 activity, the team used CRISPR-Cas9 technology. They identified POR and PGRMC1 as the two dominant genes that regulate CYP2J2 and induce the cytotoxicity of austocystin D. In addition, genomic sequencing data revealed that lysine acetyltransferase 7 (KAT7) regulated and promoting the transcription of CYP2J2. These findings provide important evidence supporting the CYP2J2-dependent cytotoxic activity of austocystin D.
“We hope that our new findings on austocystin D and its relationship with CYP2J2 may lead to the development of safe and effective therapeutic agents for cancer patients, especially those with and high levels of CYP2J2,” says Prof. Sadaie, explained how their research can be used.
Overall, the natural fungal compound austocystin D has the potential to become a game-changing drug in the fight against cancer.
***
Reference
Original paper title: Cytochrome P450 2J2 is required for natural synthesis of austocystin D to induce cancer cell toxicity.
Journal: Cancer Science
DOI: https://doi.org/10.1111/cas.16289
About Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest specialized independent scientific research university in Japan, with four campuses located in the center of Tokyo and its cities and Hokkaido. Founded in 1881, the university has contributed to Japan’s progress in science by instilling a love of science in researchers, scholars and teachers.
With the mission of “Creating science and technology for the harmonious development of nature, people and society,” TUS has conducted extensive research from basic to applied science. some of today’s most important fields TUS is a meritocracy where the best in science are recognized and recognized It is the only private university in Japan that has produced a Nobel Prize winner and the only university the only private institution in Asia to produce Nobel Prize winners within the country. department of natural sciences.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Mahito Sadaie of Tokyo University of Science
Mahito Sadaie works as a Professor in the Department of Applied Biological Sciences, Faculty of Science and Technology, Tokyo University of Science, Japan. He received his Ph.D. from the Tokyo Institute of Technology, Japan and has been actively involved in research since 2002. He specializes in cancer biology, molecular biology, cell cycle, drug discovery, and epigenetics, among other features. Over the years, he has published 21 scientific papers in leading journals that have been cited more than 2800 times.
Financial information
This work was supported by P-DIRECT from MEXT and AMED; MEXT KAKENHI (Grant Number JP19H05655) and AMED (Grant Number JP20cm0106113) to FI; JSPS KAKENHI (Grant Number JP20K07037) and research support from Yamada Science Foundation and Kobayashi Foundation to MS
Research Methodology
An experimental study
Research Topic
Cells
Article Title
Cytochrome P450 2J2 is required for the natural synthesis of austocystin D to induce cancer cell toxicity.
Publication Date of Articles
15-Jul-2024
Definition of COI
Fuyuki Ishikawa is a member of the editorial board of Cancer Science. The other authors declare no conflict of interest.
Description: AAAS and EurekAlert! are not responsible for the accuracy of the information published on EurekAlert! by participating in organizations or for the use of any information through the EurekAlert system.
#Examining #role #cytochrome #oxygenases #austocystin #Dmediated #cytotoxicity