Mapping the sexual life cycle of malaria parasites at single-cell resolution reveals genes that drive infection
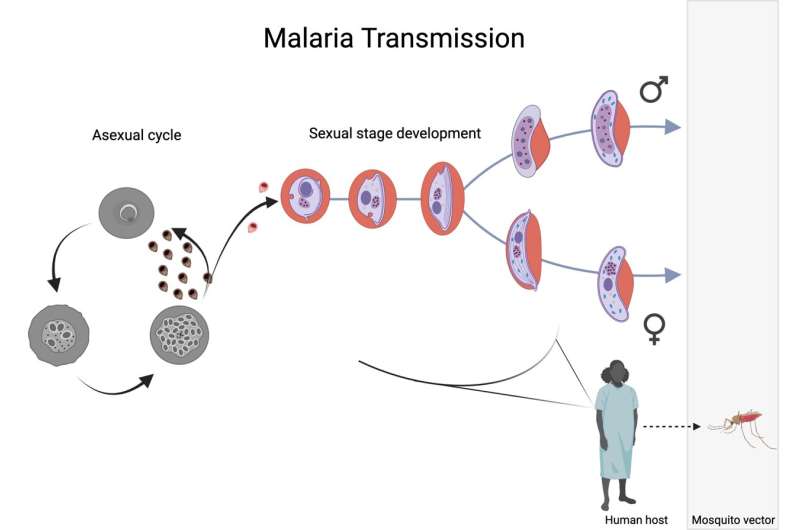
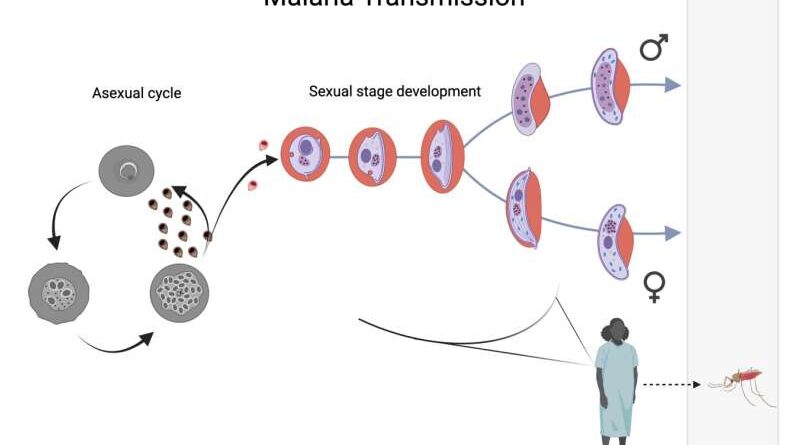
In the recipient’s blood, a small number of sexually reproducing Plasmodium falciparum parasites determine the initiation of sexual development, that is, gametocytes appear. Gametocyte development occurs in five different stages and takes about 8-12 days, which finally results in mature, stage 5, male and female gametocytes, which are suitable taken by the Anopheles mosquito. This image was created by Biorender.com. Credit: Stockholm University
Malaria is caused by a eukaryotic virus of the genus Plasmodium, and is responsible for more deaths than all other viral diseases combined. In order to pass from humans to the mosquito vector, the virus must differentiate into its sex stage, called the gametocyte stage.
Unlike the primary sex determination in mammals, which occurs at the chromosome level, it is not known what causes this unicellular virus to become male and female. New research at the University of Stockholm has used high-resolution genomic tools to map the world of genes involved in the development of gametocytes into male or female sex.
Study, published in Nature Communicationdiscovers the genes expressed in Plasmodium falciparum, the deadliest of the malaria parasites, from the beginning of sexual development to adulthood. At this stage, the male and female gametocytes are ready to be taken up by the female Anopheles mosquito to initiate the continuous process of infection.
“We combined high-throughput single-cell genetics with a new computational approach to define the expression of key gene regulators along the developmental pathway of male and female gametocytes,” says Johan Ankarklev, Assistant Professor in the Department of Molecular Biosciences, Wenner Gren Institute, and senior author of the study.
Research from Stockholm University, carried out in collaboration with Dr. Johan Henriksson at MIMS University—Umeå and the Microbial Single Cell Genomics center at SciLifeLab, are important for improving our understanding of the genes responsible for malaria transmission.
A broadly conserved family of transcription factors called ApiAP2, have emerged as important regulators of gene expression during Plasmodium lifecycle-stage differentiation and development.
“Our high level of knowledge has enabled us to correlate the expression of several types of ApiAP2 genes with the male or female lineage, which is related to their participation in the decision of the fate of sex cells. Importantly also , we have created a large group of ‘history driver.’ genes of male and female cells, which we are currently analyzing in the laboratory using CRISPR technology, “Ankarklev continues.
The study contributes important findings to the malaria community but also to the larger scientific community:
- From a clinical point of view, treatment strategies have been focusing on the blood level with many, sexual symptoms of infection, with varying success. Importantly, current treatment strategies do not prevent malaria transmission. This study provides new and important genetic markers for the future development of anti-transmission treatments, which is the only way to stop the spread of malaria.
- From an evolutionary point of view, considering that Plasmodium is an ancient eukaryote that produces both males and females, the new data and analyzes contribute new information and clues about the evolution of sex in eukaryotes.
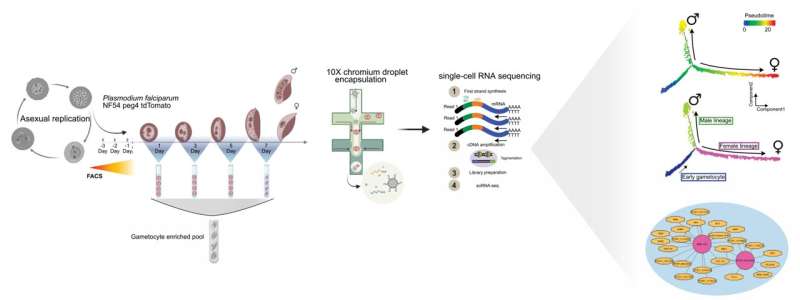
Schematic diagram showing experimental procedures used to characterize transcriptional changes during P. falciparum male and female gametocyte development, using the P. falciparum gametocyte-producing NF54 peg4-tdTomato transgenic cell line. The design highlights the development of FACS-based microbial populations followed by 10X 3′ single-cell RNA-seq (10X Genomics) and highlights two computational analyzes of the study. Credit: Stockholm University/Nat Comm graphical abstract/CC BY 4.0
Little is currently known about the sexual reproduction of malaria
Many eukaryotes perform sexual reproduction to ensure diversity and physical selection. In animals, sexual desire often affects both men and women. However, among many types of eukaryotic organisms, the systems by which sex is defined are highly variable and often obscure.
Malaria parasites, Plasmodium spp., belong to the Apicomplexan phylum, a group of invasive parasites that produce both male and female gametes. French scientist, Alphonse Laveran, first described crescent-shaped malaria in 1880. Twenty years later, British doctor, Robert Ross, discovered that malaria is transmitted by mosquitoes .
Despite these important discoveries, it is in recent years that great progress has been made to improve our understanding of the biology of the transmission stages of malaria, thanks to new and important biotechnology.
New genomic tools enable progress in malaria research
Single-cell transcriptome profiling allows a snapshot of the range of genes expressed in a single cell, in this case the malaria virus, at a specific developmental stage. When thousands of single-cell transcriptomes are included in the analysis, it becomes a powerful tool for identifying genetic pathways and developmental bifurcations, which is important for lineage tracing.
“By combining Pseudotime and RNA Velocity, two recently developed computational tools, we aligned several thousand cells along the pseudo-time axis. Second, we used RNA velocity estimates to describe the -splicing kinetics among transcripts across developmental axes allowed us to predict a large set of male and female genes, and interestingly, a large number of genes genetics has not yet been developed,” says Mubasher Mohammed, Ph.D. Ankarklev Lab student and lead author of the study.
Mohammed grew up in Sudan where he saw the devastating effects of malaria first hand.
“It’s an exciting time to be a scientist,” Mohammad says, “where new technology is helping us make leaps and bounds in our understanding of the different types of diseases that affect people.”
The transmission phase of malaria marks a significant decline in the number of infected individuals making it an attractive target for malaria control efforts.
“When such a bottleneck appears in the population, it is vulnerable to drugs and environmental conditions. By defining the molecular mechanisms of gametocyte development, we can target these pathways to develop effective mechanisms to prevent transmission, which is important for efforts to eradicate malaria,” he says. Alexis Dziedziech, former postdoc at AnkarklevLab and co-author on the study.
Additional information:
Single-cell transcriptomics reveals transcriptional programs underlying the effect of male and female cells during Plasmodium falciparum gametocytogenesis, Nature Communication (2024). DOI: 10.1038/s41467-024-51201-3. www.nature.com/articles/s41467-024-51201-3
Offered by Stockholm University
Excerpt: Mapping the sexual life cycle of malaria parasites at single-cell resolution reveals genes responsible for transmission (2024, August 26) retrieved August 26, 2024 from https:/ /phys.org/news/2024-08-sex-life-malaria-parasites-cell html
This document is subject to copyright. Except for any legitimate activity for the purpose of private study or research, no part may be reproduced without written permission. Content is provided for informational purposes only.
#Mapping #sexual #life #cycle #malaria #parasites #singlecell #resolution #reveals #genes #drive #infection